Shopping cart
Hello There!
Welcome to GattPrep, your Guide for Life and Learning
👩🏽🏫 Introduction
Hello again, scientist-in-training! 👋🏽
Have you ever tried to wash with soap and found it wouldn’t lather properly? That may be because the water you’re using is hard. Understanding the difference between hard and soft water is not just a science topic—it’s a daily life issue that affects everything from bathing and cleaning to industrial processes.
By the end of this lesson, you’ll know exactly what makes water hard or soft, why it matters, and how we can change it.
📘 Core Concepts
Hard water is water that contains dissolved calcium (Ca²⁺), magnesium (Mg²⁺), or sometimes iron (Fe²⁺) ions. These minerals enter water when it flows through rocks like limestone or chalk.
| Type of Hardness | Description |
|---|---|
| Temporary Hardness | Caused by dissolved bicarbonates of calcium or magnesium. Can be removed by boiling. |
| Permanent Hardness | Caused by dissolved sulfates or chlorides. Cannot be removed by boiling. |
Soft water lacks these hardness-causing minerals. It forms lather easily with soap and doesn’t leave scum or scale in pipes or kettles.
🔍 Comparison Table
| Feature | Hard Water | Soft Water |
|---|---|---|
| Soap Use | Forms scum, hard to lather | Lathers easily |
| Reaction with heat | Forms scale in kettles/boilers | No scale formation |
| Minerals Present | Ca²⁺, Mg²⁺, sometimes Fe²⁺ | Few or no dissolved minerals |
| Taste | Often has a distinct mineral taste | Generally tasteless |
🧪 Causes of Hardness in Water
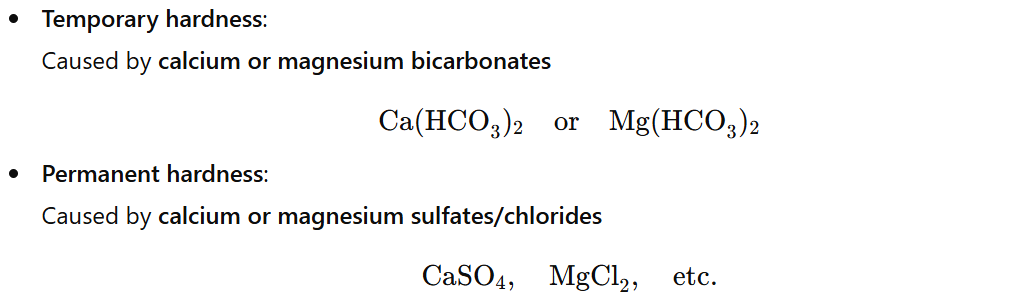
👨🏽🔬 How to Remove Hardness
| Method | Works On | How It Works |
|---|---|---|
| Boiling | Temporary hardness | Heat breaks bicarbonates into carbonates + CO₂ |
| Adding washing soda (Na₂CO₃) | Both types | Reacts with Ca²⁺/Mg²⁺ to form insoluble carbonates |
| Ion-exchange (resin) | Both types | Swaps Ca²⁺/Mg²⁺ with Na⁺ ions in special filters |
| Distillation | Both types | Boils and re-condenses pure water, leaving salts |
🧪 Example (Boiling to Remove Temporary Hardness):
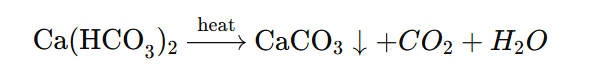
(Calcium carbonate precipitates out as a solid.)
📐 Sample Problem Walkthrough
🧠 Problem: You are asked to test a sample of water for hardness and remove it. What steps would you take?
✅ Step-by-Step Solution:
Test for hardness by adding soap to the water and shaking. If no lather forms, it is hard water.
Boil the water. If lather forms afterward, it had temporary hardness.
If boiling doesn’t help, add washing soda or use ion-exchange resin to remove permanent hardness.
✔️ Answer: Test with soap, boil to check for temporary hardness, and treat with chemicals or ion exchange if permanent hardness remains.
✍🏽 Practice Exercises
1. Fill in the blanks
(a) Water that contains dissolved calcium and magnesium ions is called ______ water.
(b) ______ hardness can be removed by boiling, but ______ hardness cannot.
✅ Answers:
(a) hard
(b) Temporary; permanent
2. True or False
(a) Permanent hardness is caused by bicarbonates. (False)
(b) Soft water forms lather easily with soap. (True)
3. Short Answer
Explain how washing soda softens hard water.
✅ Sample Answer:
Washing soda (sodium carbonate) reacts with calcium and magnesium ions in hard water to form insoluble carbonates that precipitate out, leaving soft water behind.
🌀 Recap
Today you learned:
Hard water contains minerals like calcium and magnesium.
There are two types: temporary (removable by boiling) and permanent (needs chemical treatment).
Hardness affects daily activities like washing and industrial use.
We can soften water using boiling, washing soda, ion exchange, or distillation.
💭 Reflection Prompt
Next time you’re doing laundry or boiling water for tea, ask yourself: Is the water you’re using hard or soft? How can you tell? Write down two methods to test and soften water in your environment.